|
|
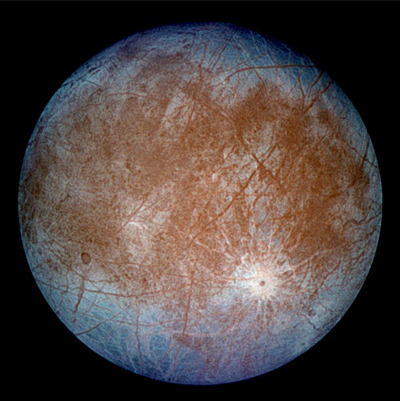
Jupiter’s moon Europa |
This meditation is an extension of a series of Facebook posts around the question of what conditions are necessary for the development of life, which itself is an extension of the Drake equation for estimating the probability of finding other life and civilizations in the universe. The proposer, William Maness of my Facebook acquaintance, posted: “Let’s go the other way. Let’s say that Earth’s condition is astonishingly rare. How rare does it have to be to be the only one in the galaxy. How rare to be the only one in the universe?”
And then he proposed conditions for life on Earth as we know it: strong magnetic field, stable sun, Goldilocks zone (meaning both the right part of the galaxy, in terms of density of nearby stars and their radiation, as well the solar system’s “habitable zone,” with planetary temperatures that can support liquid water), a large companion body (to create tides, which set a pattern of inundation and exposure for sea life at the edge of the land, among other things), no gamma emitters nearby, debris-cleared orbit (to minimize life-killing asteroid impacts), abundant liquid water, no conditions that kill carbon life in said ocean, an active lithosphere (with plate tectonics to renew the surface, replenish the atmosphere, and relieve geothermal stresses1), an active water cycle, and a transparent atmosphere. “These are just a few that come to mind,” he wrote.
My first response was to say that some of these conditions overlap and work to the same purpose. For example, the conditions of having a strong magnetic field and a stable sun are related, as their result is to protect developing and existing life from the solar wind and radiation bursts. Having no nearby gamma emitters is part of that requirement, too. But note that if your definition of life includes—or is excluded to—cockroaches and tardigrades, which seem not to care much about hard radiation, these several requirements may not be absolute.
Having liquid water and an abundance of carbon are nice. But as I’ve noted elsewhere,2 you could construct a parallel DNA chemistry from silicon and arsenic. The silicon atom has the same chemical-bonding valence as carbon, while arsenic has the same valence as phosphorus. So silicon might replace the carbon atoms in the ribose rings and the purines and pyrimidines that are the main features of DNA and RNA molecules. And arsenic might replace the phosphorus atoms in the bonds that connect those ribose rings into a long-chain polymer. The resulting molecules would be heavier, of course, having a higher aggregate atomic weight. And they would be somewhat more fragile, because their traded electrons would occupy a higher electron orbit. But these replicant molecules would still function like carbon-based DNA.
And liquid water does have some unique properties. The water molecule is easily dissociated into its component oxygen and hydrogen atoms. The molecule has an asymmetrical arrangement, placing the two hydrogen atoms at sixty degrees apart on one side of the oxygen atom, creating a positive and negative side to each molecule. This arrangement allows other molecules to be either “hydrophilic” and attracted to water or “hydrophobic” and repel water. Water as a fluid is also relatively incompressible—you can’t squeeze it in its liquid phase—so that the water in a deep lake or ocean doesn’t get thicker and sludgier as you descend, becoming paste-like or semi-solid. Instead, the pressure just increases while the density remains the same. These features create an important condition for life forms like Earth’s sea creatures, who are composed of mostly water themselves, metabolize the dissociated oxygen in water, and range freely from the surface to the deeps.
That angular separation on the water molecule forces it to form a hexagonal crystal when frozen, so that the solid phase is actually less dense than the liquid phase, enabling it to float. If solid water sank to the bottom of a pond or ocean, where temperatures are generally cooler, then a temporary drop in ambient temperature might freeze any body of water solid. And there it would stay frozen for who knows how long—not until next summer but more likely until the next extreme in the climate cycle.
But other liquids with a low chemical reactivity and low compressibility could support life almost as well as water does—although it would be chemically and physically different from ours and might prefer different ambient conditions.
Other planetary features like a large companion (for tides) and active lithosphere (for plate tectonics and volcanoes) are only required for the kind of life we recognize. I’m betting that, when we find life out there among the stars, it will surprise us. But that wasn’t the premise of the question as originally posed, which acknowledged that it was working backward (i.e., “going the other way”): What kind of conditions will produce us, the life that we know and recognize? And that may be too limiting a definition.
We can begin as a given that the same laws of physics and chemistry exist elsewhere throughout the universe. Go to any other star with a planet, and you’ll find the same atoms from our Periodic Table—although not necessarily in the same abundance and distribution. They will tend to form similar molecules—although perhaps with different underlying chemical reactions having different, temperature-dependent endo- and exothermic requirements—and so the abundance and distribution of life-creating or life-destroying substances will depend on local conditions. The gravity curve will follow the equations we use to measure it here on Earth—although the resulting values will necessarily be different, based on solar and planetary density and distance. The physics of electromagnetism and radiation will apply—although the quality of the light and its effects on biochemistry and biodiversity will be different, based on the output of the local star.
The nature of life, however defined, is that is evolves in and adapts to the environment it finds. Otherwise, whatever you find on a new planet is just an artifact or an exception. This presumes, of course, that evolution is present on the planet and is based on either a system of replicating molecules, similar but not necessarily identical to Earth’s DNA-RNA-protein coding system.3 Once the principle of replication-with-modification becomes established and gives rise to “life,” it will already be adapted to the conditions that it finds and then change itself as they change.
This evolution will be able to give rise to organisms that are not like us either physically or chemically. Even on Earth, and working under the DNA-RNA-protein coding system, we can find life that is strange and different. Consider the organisms that our deep-ocean searches have discovered clinging to the sides of undersea volcanic vents: adapted to total darkness and huge surface pressures, tolerating the extreme temperatures of superheated water, and metabolizing sulfur compounds instead of carbohydrates. The life that we recognize from this planet’s surface was able to descend and adapt to that hell. Or rather, our kind of life didn’t adapt itself: any of its great-great-grandchildren who happened to survive because of compounding genetic mutations became able to thrive under those conditions. Remember that the original life on Earth evolved in a carbon dioxide–rich atmosphere. Then plants began metabolizing that carbon in a photosynthetic reaction driven by sunlight and released free oxygen into the atmosphere. Only then did later organisms—“our” kind of life which moves, wiggles, walks, and talks—adapt to breathe and metabolize that oxygen.
As for what conditions might be required to create life, consider the smallest of the Galilean moons, Europa. Jupiter is not in the Sun’s “habitable zone,” with temperatures that generally keep water a liquid. Still, Europa is suspected of having an ocean under its icy shell that is kept warm by tidal flexing in its orbit around the giant planet. The ocean under the ice might contain life, protected not by a thick atmosphere and planetary magnetic field, as on Earth, but by the layers of ice themselves, because water is a good shield against radiation.4 Whatever life develops in this ocean would be different from ours—not based on or even seeing the Sun’s light, with no possibility of moving out onto land and developing the things we humans cherish, like fire, metals, and radio and television. But it would still be life under conditions that do not entirely match those on Earth.
When we get out among the stars, we’re going to have to expand our definition of life exponentially. I suspect that will quickly turn our teaching of biology—and so much else—on its head.
1. If you think geothermal stress isn’t important, consider Earth’s sister planet, Venus. By studying the uniformly limited number and apparent recent age of the impact craters on the surface, astronomers have determined that Venus must lack a system of plate tectonics, with its corresponding subduction of surface layers and creation of volcanic hot spots that release core heat, as on Earth. Instead, the planet appears to go through periodic renewals, where the entire surface melts from within and then resolidifies. That would be bad for any life trying to gain a foothold on the rocks there.
2. See The God Molecule from May 28, 2017.
3. For example, a machine-based organism that was able to sample its environment and rewrite its underlying operating code to thrive under those conditions would be a similar but different analog of our biological kind of life. For that matter, you might consider our molecular form of life as simply a kind of nanotechnology.
4. When I was in college, I had a roommate who worked as shift operator at the university’s TRIGA reactor. This was one of those “swimming pool” reactors, used for research, training, and experiments with radiation. When he took me on a tour, we stood at the railing and looked directly down at the reactor core, which when operating glowed with the beautiful blue light of Cherenkov radiation. I pointed at the active core and asked my roommate, “Why am I not dead?” He replied that the twenty feet of water between us and the radiation flux with its fast neutrons was better protection than a foot of lead shielding. I then saw bubbles of gas rising from the reactor and bursting on the surface about eight feet away. I asked what it was, and he said it was a radioactive isotope of oxygen. “Why am I not dead?” Because that isotope possesses a half-life of eight seconds and had mostly decayed to regular oxygen by the time it reached the surface, where any residual isotope dissipated into the room’s atmosphere before decaying further.


