|
|
The Buckminsterfullerene |
In the movie Star Trek IV: The Voyage Home, Scott and McCoy try to find a light and strong material with which to build a giant seawater tank in the hold of their stolen Klingon ship. They locate a manufacturer of plexiglass in 20th-century San Francisco and offer him the formula for “transparent aluminum,” a material from the 23rd century. They assuage their consciences about temporal paradoxes by suggesting, “How do we know he didn’t invent it?”
Well, he didn’t. The crystal in many of today’s quality watches of all descriptions, including my upgraded Apple Watch, are made from synthetic sapphire. Since the composition of sapphire is corundum, or crystalline aluminum oxide (Al2O3)—the same material from which, in powder form, metallic aluminum is smelted—along with traces of iron, titanium, chromium, vanadium, or magnesium depending on the gem’s color,1 you could easily say that this crystal, which is durable, lightweight, strong, and scratch-resistant, is indeed “transparent aluminum.”
Synthetic rubies and then sapphires were invented in 1902 by French chemist Auguste Vermeuil. He deposited the requisite chemicals in the requisite combinations on a ceramic base by heating and passing them through a hydrogen-oxygen flame, then increasing the temperature to the point of melting and crystallizing the alumina. So far, we can make watch crystals and synthetic gemstones with this process. Whether it is scalable for fabricating whole spaceships is another question. But the technology is young yet.
If you are a dedicated browser among the pages of Science and Nature, as I am, with forays into Scientific American and Popular Science, you know that the world of materials science is hot right now. And the element carbon is getting a resurgence—but not as a fuel.
Carbon has the happy ability to bond with many different atoms including, sometimes, itself. Its four covalent bonding points allow it to share single, double, and even triple bonds with other carbon atoms, often forming chains and hexagonal rings that are the building blocks of organic chemistry and so the basis of all life on this planet. These rings and chains leave room for adding other atoms and whole other molecules, making carbon the backbone of the chemical world’s Swiss Army knife.
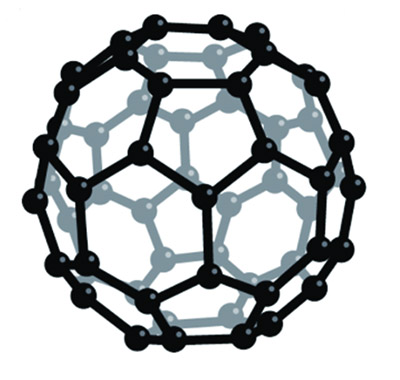
What modern materials scientists have discovered is that bonding among carbon atoms can be induced in several structural forms. We are all familiar with the three-dimensional, tetrahedral-shaped crystal of a diamond, whose bonds are so strong that they make it one of the hardest materials known. But those atoms can also be knit into fibers, which are then stabilized and supported in an epoxy resin to create a material that is light, strong, and useful in many applications, sometimes replacing steel. The carbon atoms call also form two-dimensional, hexagonal structures that can also be laid out in endless sheets, called graphene, which are strong and supple even at one molecule’s thickness.2 Or smaller sections of those sheets can be bent into nano-scale tubules, which are even stronger than the carbon fibers and have interesting chemical uses. And finally, the carbon atoms can be joined into microscopic soccer ball–like molecules, made of twenty hexagons and twelve pentagons with the formula C60 (pictured). This is the buckminsterfullerene—named after the architect Buckminster Fuller, who invented a spherical structure of similar configuration.
Graphene is not only strong but it is also electrically and thermally conductive, useful for dissipating heat. It has a high surface-to-volume ratio, which means it can be used to make batteries and fuel cells more efficient. It holds promise for flexible display screens and solar photovoltaic cells. And as an additive to paint and in other surface preparations, it can increase wear and resistance to corrosion.
Carbon nanotubes, which are also electromagnetically conductive, can be used in radio antennas and as the brushes in electric motors. Being biodegradable, they can be used in tissue engineering for bone, cartilage, and muscle. Because they are easily absorbed into cells, they can carry other molecules such as medicines as well as protein and DNA therapies. Spun into yard, the tubes would offer superior strength and wear in clothing, sports gear, combat armor, and even in cables for bridges and for space elevators—imaginative projects that have been proposed for hauling people and cargo up to geosynchronous orbit.
Buckyballs have potential uses as a drug delivery system, as lubricants that will resist breaking down under wear and heat, and as catalysts in chemical reactions. As a medicine in itself, the C60 fullerene can be used as an antioxidant, because it reacts with free radicals.
And that is just some of the potential for various pure forms of carbon.
Work on the genetics of plants and animals other than humans will have far reaching effects, too, in terms of bio-simulates. For example, spiders produce a raw silk that they spin into a strand which has a tensile strength greater than steel and more fracture-resistance than the aramid fibers used in Kevlar body armor.3 We could farm spiders for this silk, the way we do silkworms for their cocoon fibers, except that spiders in captivity will eat each other. But several companies are now working on creating synthetic spider silk.
Another area ripe for development is natural latex, the basis of all our rubber products. Rubber trees are native to South America, where they naturally grow in splendid isolation because a fungus-based leaf blight destroys any trees that grow too close together. Attempts by Ford to create a rubber plantation in Brazil in the late 1920s failed because of this blight. All of the world’s rubber currently comes from trees grown on plantations in Southeast Asia, where they survive only with the strictest vigilance—cutting and burning whole plantations at the first sign of blight—and government control of imported plants and vegetables.
Natural rubber is essential to modern life. Synthetics based on petroleum chemistry, like styrene-butadiene, are less resilient and elastic. A natural rubber tire can thaw from being frozen in the wheel well of an airliner at 35,000 feet in the time it takes for the plane to descend and land, while a synthetic-based tire will remain frozen and shatter upon impact. So discovering a genetic formula for latex and being able to extrude it in the same way the rubber tree weeps its sap would be a godsend.
One of the unsung stories of our modern life is the nature of our materials. They are not just getting cheaper but also lighter, stronger, and better. And this is only the beginning.
1. Just about every color but red. And a red crystal of virtually the same composition is called a ruby.
Emeralds are a different material, however, based on beryl, which is composed of beryllium aluminum silicate (Be3Al2Si6O18) in hexagonal crystals with traces of chromium and vanadium.
2. The graphite in pencil “leads” is not chemically lead but a pure form of carbon. Small bits of what we now call graphene are layered into a three-dimensional composite, like the layers in sandstone or shale.
3. Interestingly, spiders that are fed a diet of carbon nanotubes make a silk that is even stronger, incorporating the tubules into its protein microfibers.
No comments:
Post a Comment